By Aina J. Khan, The New York Times
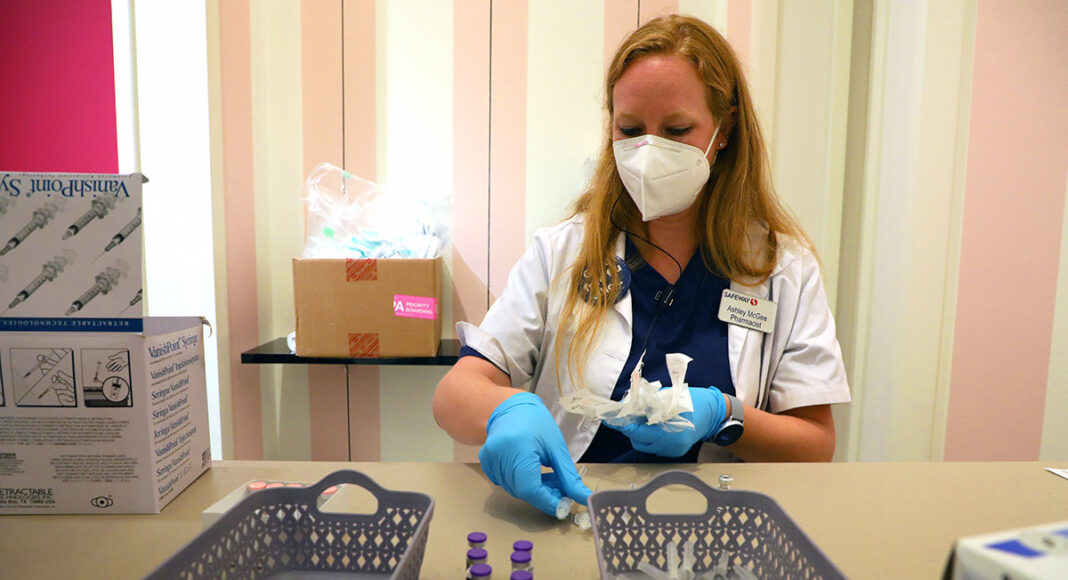
Pfizer reported data Friday showing that its coronavirus vaccine had a 90.7% efficacy rate in preventing symptomatic COVID-19 in a clinical trial of children ages 5 to 11.
The company and its partner BioNTech submitted the information to the Food and Drug Administration, which was expected to release its own analysis of the data later in the day.
Children in the trial received a dose of 10 micrograms, one-third of the adult dose. Researchers said that the dosage was safe and that trial participants had seen only mild side effects.
Of 2,268 children in the trial, twice as many were given the vaccine as received a placebo. Sixteen children who received the placebo got COVID-19, compared with three who received the vaccine.
There were no cases of severe illness. All of the cases occurred in July or later, a time when the highly transmissible delta variant was spreading in the United States and globally, Pfizer said.
After the second shot, the children had levels of neutralizing antibodies at least equal to those of 16-to-25-year-old volunteers in another Pfizer-BioNTech trial. Although antibody levels are just one measure of the immune system’s response, experts have said such a finding would indicate that one-third strength was the proper dosage for young children.
Pfizer also reported that there were no cases of the heart conditions of myocarditis, which is inflammation of the heart muscle, or pericarditis, which is inflammation of the lining around the heart, in volunteers roughly three months after receiving the second dose. Both conditions have been tied to adult recipients of the vaccine, particularly younger men. Because the conditions are rare, it is unlikely that one of them would have occurred in a study size as small as Pfizer’s.
Studies have also shown that the risk of heart problems after COVID-19 is higher than after vaccination.
The FDA released the data before a Tuesday meeting at which the agency’s expert advisers will decide whether to recommend that the agency authorize the vaccine for children in this age group. Federal regulators have already made the vaccine available for those 12 and older.
The Biden administration has been eagerly promoting the prospect of protection for the 28 million children aged 5 to 11, promising that tens of thousands of pediatric or primary care offices, pharmacies, and school and rural health clinics will be ready to administer shots if regulators grant authorization. With the school year well underway, many parents are anxiously awaiting the development.
If the FDA grants authorization, distribution of the doses would still depend on signoff by the Centers for Disease Control and Prevention, which sets vaccine policy. The CDC has scheduled a two-day meeting of its own advisory committee for Nov. 2 and 3. Federal officials have said they intend to ship 15 million doses to the states immediately if regulatory and health officials authorize the move.
About 17 million adolescents aged 12 to 15 became eligible for the Pfizer-BioNTech vaccine in May. A month ago, Pfizer and BioNTech said their vaccine was also safe and effective for young children but did not release any detailed clinical trial data.
This article originally appeared in The New York Times.